Type 1 diabetes (T1D) is a chronic autoimmune disease, leading to the destruction of β-cell mass causing insulin deficiency and poor glycaemic control. While insulin therapy prolongs survival, it fails to replicate endogenous insulin dynamics. Transplant-based approaches are constrained by donor scarcity and lifelong immunosuppression. To address this unmet need, we are investigating the fundamental epigenetic mechanisms that constrain β-cell gene activation in non-endocrine pancreatic cells. In the pancreas, the epigenetic silencing mechanism mediated by the Polycomb Repressive Complex 2 (PRC2) restricts the activation of β-cell gene networks in non-endocrine cell types, such as ductal cells. PRC2, through its catalytic subunits EZH1 and EZH2, plays a central role in maintaining transcriptional repression by catalysing the trimethylation of histone H3 at lysine 27 (H3K27me3). EZH2 is the primary catalytic subunit of PRC2 during early development and in proliferative cells, whereas EZH1 predominates in more differentiated and quiescent adult tissues, including the pancreas. Although EZH1 exerts weaker methyltransferase activity, it plays a critical compensatory role in maintaining repressive chromatin states. This functional redundancy presents a barrier to β-cell gene activation, as inhibition of EZH2 alone may be insufficient to overcome PRC2-mediated repression in adult ductal cells. Disentangling the distinct versus overlapping roles of EZH1 and EZH2 is therefore essential to define the threshold for effective epigenetic reprogramming.
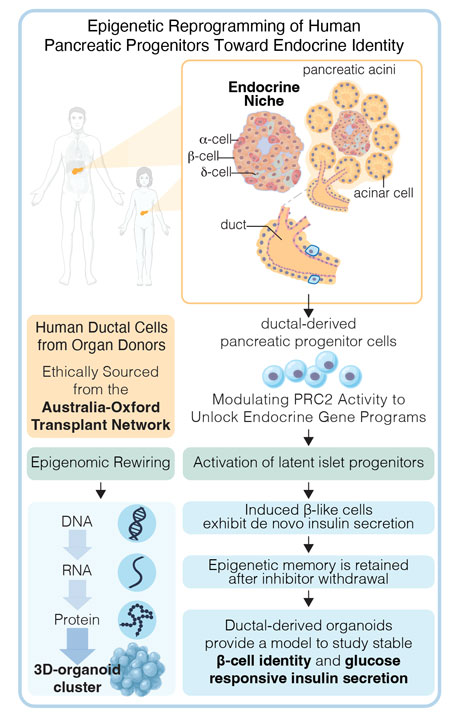 Schematic overview of our human in vitro system for studying the epigenetic regulation of β-cell gene programs. PRC2 modulation via EZH1/2 inhibition enables chromatin opening and endocrine gene expression in pancreatic ductal cells. This platform supports investigation of reprogramming trajectories, stability, and molecular memory in the absence of genetic engineering.
Schematic overview of our human in vitro system for studying the epigenetic regulation of β-cell gene programs. PRC2 modulation via EZH1/2 inhibition enables chromatin opening and endocrine gene expression in pancreatic ductal cells. This platform supports investigation of reprogramming trajectories, stability, and molecular memory in the absence of genetic engineering.
The overall objective is to delineate the molecular basis, cellular dynamics, and functional durability of pancreatic ductal cell reprogramming induced by selective versus dual inhibition of EZH1 and EZH2 — two subunits of PRC2 with distinct roles in chromatin repression. By comparing their individual and combined effects on β-cell gene activation, we aim to define the epigenetic requirements for stable lineage conversion. We have extensively characterised the epigenetic mechanisms driving the reprogramming of pancreatic ductal progenitor cells into insulin-producing β-cells. Our group has made foundational contributions to this field. These seminal discoveries form the cornerstone of our regenerative pipeline and position our team as international leaders in β-cell programming and pancreatic cell plasticity.
This project will generate foundational insights into:
- The distinct roles of EZH1 and EZH2 in maintaining transcriptional repression of β-cell gene networks in adult human pancreatic cells.
- Mechanistic pathways by which epigenetic derepression unlocks latent endocrine potential in non-β pancreatic lineages.
- Single-cell maps of ductal subpopulations with reprogramming competence, including trajectory models and regulatory circuit analysis.
- Functional benchmarks for β-like cell induction and stability, including chromatin memory and lineage persistence after compound withdrawal.
- Organoid-based human models for probing endocrine reprogramming under defined epigenetic modulation, enabling scalable and reproducible discovery in human tissue systems.
- Predictive markers and reprogramming-associated chromatin features that can inform future biomarker development or in vivo validation strategies.