The recent discovery that thousands of RNAs are transcribed by the cell that fail to code for protein, highlights a significant gap in our understanding of transcriptional control of cellular function. The extent of this phenomenon is highlighted by the fact that over 75 per cent of the human genome is transcribed into RNA, but only approximately 2 per cent is translated into known proteins. This raises the question as to what function the other so-called 'non-coding RNAs' (ncRNAs) are performing. To answer this question, an enormous amount of research has been performed over the past decade to identify several classes of ncRNAs, predominantly short ncRNAs (<200 nucleotides), that have been confirmed to have functional biological significance. This has been particularly evident by the paradigm shifting work performed in the microRNA field. More recently, significant advances in sequencing technology and bioinformatics has allowed for the identification of other classes of ncRNAs, particularly those termed long ncRNA (lncRNA, >200 nucleotides long) that often have a similar structure to protein coding messenger RNAs (mRNA). Studies have estimated there to be possibly >50,000 lncRNAs expressed from the human genome of which very few have been identified and characterised. Data from the few that have been studied, suggests that lncRNAs play a pivotal role in developmental processes including chromatin remodelling, transcriptional activity and sequestration of other RNA species. Indeed, several lncRNAs have been associated with tissue development and disease, particularly in cell types that undergo differentiation including post-mitotic cells such as neurons and striated muscle.
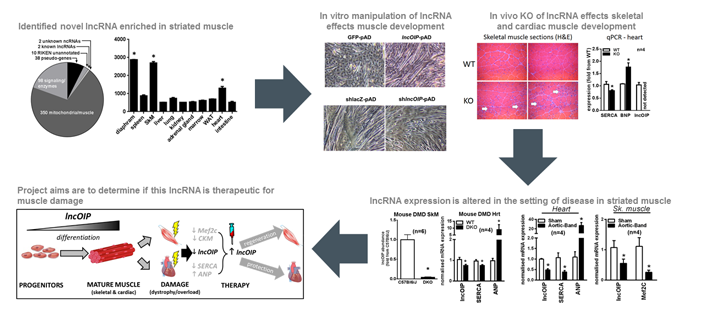
Of particular interest to this application, is the role of lncRNAs in the development of striated muscle (skeletal and cardiac). Not only have specific lncRNAs been shown to functionally regulate developmental programs in muscle, but their expression is altered in disease settings such as muscular dystrophy and heart failure. Moreover, manipulating these lncRNAs during disease was associated with functional improvement in phenotypic end-points. Thus, lncRNAs forge a new frontier in the search for therapies that treat diseases of muscle dysfunction, where currently very few treatment options are available. This study will focus on a novel lncRNA called lncOIP, which we identified to be intimately associated with striated muscle development and disease. We will extend on our preliminary finding that lncOIP is a key regulator of striated muscle development test if reintroducing lncOIP can aid regeneration of skeletal muscle, and reduce disease severity in pathological cardiac hypertrophy.