About the Molecular Metabolism and Ageing laboratory
Cardiometabolic diseases including obesity, diabetes and cardiovascular disease are often considered to be heterogeneous, polygenic diseases. That is, these conditions develop as a result of numerous different environmental and genetic causes. However, ultimately these collectively lead to disruption of a common set of pathways that manifests a similar disease. Several decades of research has been dedicated to determining what these common set of pathways are, with significant amounts of progress having been made. Our group has an interest in several of the well documented pathways identified, however much of our focus centres on the more recently identified dysfunction of mitochondrial activity.
Found in almost every cell type in our body, mitochondria are organelles whose primary function is to utilise glucose (sugar) and fatty acids (fats) to provide the cell with energy (ATP). Healthy mitochondria are more efficient and therefore many cell types, particularly post-mitotic cells such as muscle and neurons, have developed unique ways to maintain a healthy pool of mitochondria. This includes specific mechanisms to degrade old or damaged mitochondria (mitophagy) or to generate new mitochondria when necessary (biogenesis). However, the molecular networks that control efficient mitochondrial health, and indeed the cross-talk between them, are poorly characterised. Moreover, even less is known about how these mitochondrial molecular networks are effected by, or causative for cardiometabolic disease. Therefore, our studies aim to elucidate key pathways involved in mitochondrial function, energy metabolism and tissue health with the aim of determining how they can be therapeutically manipulated to prevent or treat disease and ageing.
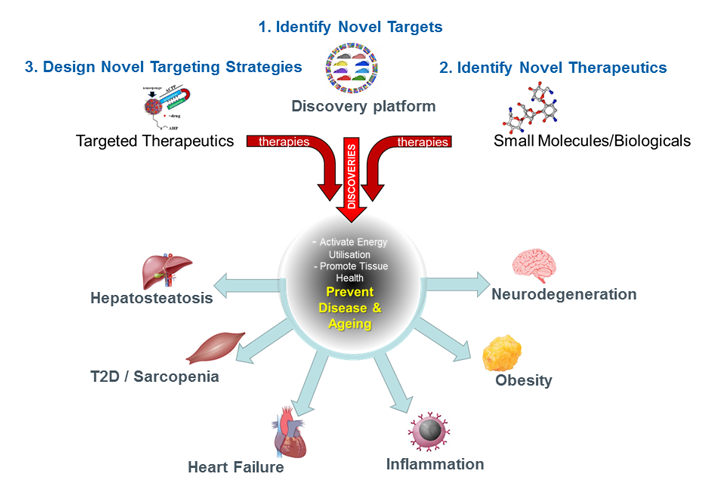
Research focus
- Diabetes, obesity, heart failure, liver disease, neurodegeneration.
- Integration of cutting-edge technologies and systems biology for the identification of novel regulators of mitochondrial function and energy metabolism.
- Determine if genetic and pharmacological manipulation of mitochondrial health can prevent or reverse cardiometabolic disease and ageing.
- Design and validation of targeted nanoparticles for the delivery of novel therapeutics.
- Identification and validation of novel biomarkers and diagnostics for cardiometabolic disease and ageing.
- Long non-coding (lnc)RNAs in cardiometabolic disease.














